Latex-Fruit Syndrome and Class 2 Food Allergy
[ English | Japanese ]
 It is estimated that 50-70 % of latex-allergic people have IgE antibodies cross-reactive to the antigens coming from some vegetable foods [1]. In addition, increasing numbers of plant-derived foods are suspected to cross-react with latex antigens. Fruits are especially notorious for their frequent cross-reactivity, which is referred to as "latex-fruit syndrome"[2,3]. Besides various plant-derived foods, latex-allergic people are occasionally affected by some kinds of pollen and medical plants. This extensive cross-reactivity of latex-allergic patients is explained mostly by the major latex allergens are being pathogenesis-, or rather, defense-related proteins of a rubber tree. Higher plants universally
induce such protective proteins under certain conditions, and their structures are relatively conserved during the course of evolution. These features of defense-related proteins provide common epitopes for IgE antibodies [4]. In other words, it is considered that the defense-related proteins of the rubber tree and of cross-reactive plants act as pan-allergens responsible for the extensive cross-reactivity of latex-sensitized people.
It is estimated that 50-70 % of latex-allergic people have IgE antibodies cross-reactive to the antigens coming from some vegetable foods [1]. In addition, increasing numbers of plant-derived foods are suspected to cross-react with latex antigens. Fruits are especially notorious for their frequent cross-reactivity, which is referred to as "latex-fruit syndrome"[2,3]. Besides various plant-derived foods, latex-allergic people are occasionally affected by some kinds of pollen and medical plants. This extensive cross-reactivity of latex-allergic patients is explained mostly by the major latex allergens are being pathogenesis-, or rather, defense-related proteins of a rubber tree. Higher plants universally
induce such protective proteins under certain conditions, and their structures are relatively conserved during the course of evolution. These features of defense-related proteins provide common epitopes for IgE antibodies [4]. In other words, it is considered that the defense-related proteins of the rubber tree and of cross-reactive plants act as pan-allergens responsible for the extensive cross-reactivity of latex-sensitized people.
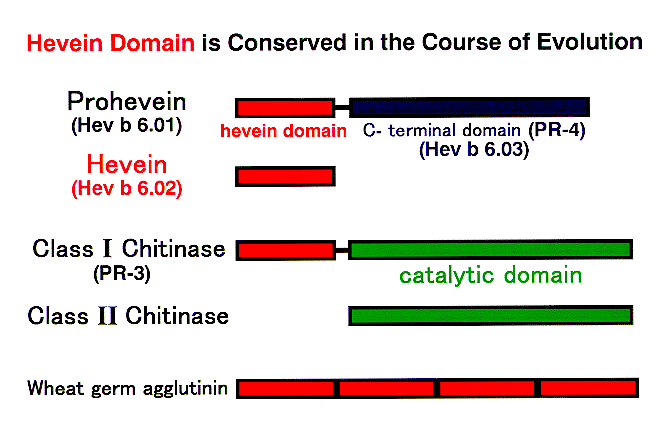
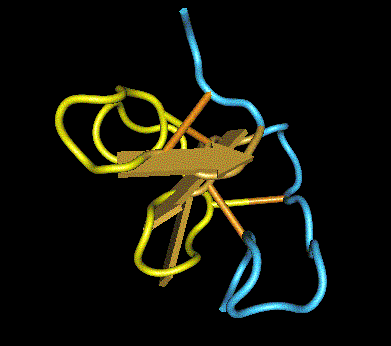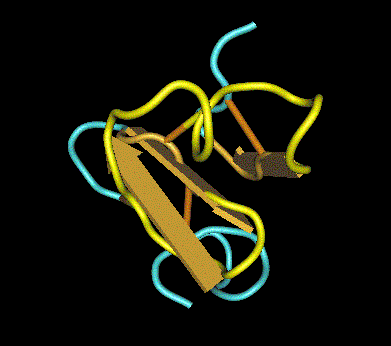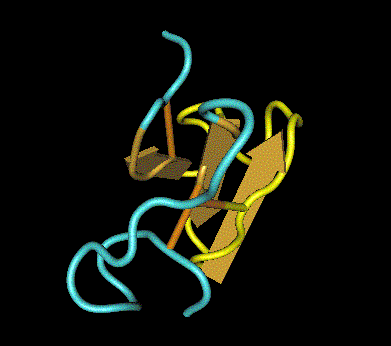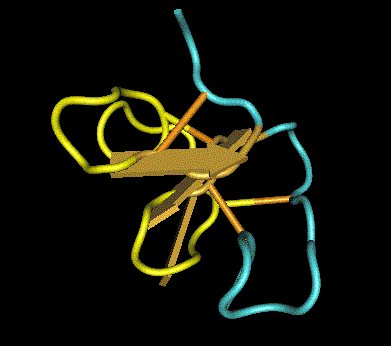 In support of this assumption, the class I chitinase of avocado, chestnut, and banana, which belong to a family of pathogenesis-related proteins (PR-3), was revealed to be an important cross-reactive allergen for latex-sensitized people [5]. Class I chitinase of plants contains a hevein-like domain in common at the N-terminus (Fig. 1). Hevein (Hev b 6.02) is one of the most important latex allergens. Thus, the cross-reactivity of latex-allergic patients can be partly ascribed to the shared hevein-related structures (Fig. 1). The three-dimensional structure of hevein is shown in Fig. 2. The yellow portion indicates the linear IgE epitopes. This site closely overlaps the conserved residues indispensable to the chitin-binding
property of hevein, which is the root of its anti-fungal activity. On the other hand, patatin has been identified as a major cross-reactive allergen in potato. Patatin is a defense-related protein with lipid acyl-hydrolase or esterase activity, and functions as an important storage protein in potato tuber. Latex allergens (Hev b 7) homologous to patatin have also been found in natural rubber and have been officially registered. As in the case of cross-reactivity between class I chitinase in vegetables and hevein, the structural similarity between patatin and Hev b 7 likely accounts for part of the cross-reactivity of latex-sensitized people. [5]
In support of this assumption, the class I chitinase of avocado, chestnut, and banana, which belong to a family of pathogenesis-related proteins (PR-3), was revealed to be an important cross-reactive allergen for latex-sensitized people [5]. Class I chitinase of plants contains a hevein-like domain in common at the N-terminus (Fig. 1). Hevein (Hev b 6.02) is one of the most important latex allergens. Thus, the cross-reactivity of latex-allergic patients can be partly ascribed to the shared hevein-related structures (Fig. 1). The three-dimensional structure of hevein is shown in Fig. 2. The yellow portion indicates the linear IgE epitopes. This site closely overlaps the conserved residues indispensable to the chitin-binding
property of hevein, which is the root of its anti-fungal activity. On the other hand, patatin has been identified as a major cross-reactive allergen in potato. Patatin is a defense-related protein with lipid acyl-hydrolase or esterase activity, and functions as an important storage protein in potato tuber. Latex allergens (Hev b 7) homologous to patatin have also been found in natural rubber and have been officially registered. As in the case of cross-reactivity between class I chitinase in vegetables and hevein, the structural similarity between patatin and Hev b 7 likely accounts for part of the cross-reactivity of latex-sensitized people. [5]
The establishment of latex-fruit syndrome is comparable with that of pollen-food allergy syndrome, where patients suffering from pollinosis also experience food allergies ranging from itching and pruritus around the oral cavity (oral allergy syndrome: OAS) to generalized urticaria and even anaphylaxis. In the pollen-food allergy syndrome, a patient is fist sensitized by inhaling antigens concomitant with pollen. Thereafter, immediate-type symptoms around the oral cavity (OAS) begin to be provoked when the patient eats any plant-derived foods containing proteins cross-reactive to the sensitizing antigen. According to our conventional understanding of a food allergy, sensitization is established by an abnormally stable food
protein ingested per-orally. After the completion of per-oral sensitization, allergic symptoms can be provoked whenever the patient eats the same protein. In this traditional concept of a food allergy, a key point is that the same antigen plays a vital role both in the sensitization process and the symptom-elicitation process. It has long been believed that only special food proteins stable to heat treatments and resistant to digestive enzymes can become food allergens. This explanation seems to be based on the idea that only such a special protein can reach the intestine without being fragmented and thus establish per-oral sensitization. Recently, a food allergy following this traditional concept has been called "class 1 food allergy" (or type I food allergy). In addition, food proteins responsible for such food allergies are now being
called "complete food allergens" or "class 1 food allergens"(Fig. 3, left) [7]. A complete food allergen is an antigen having the capacity for both per-oral sensitization and per-oral elicitation.
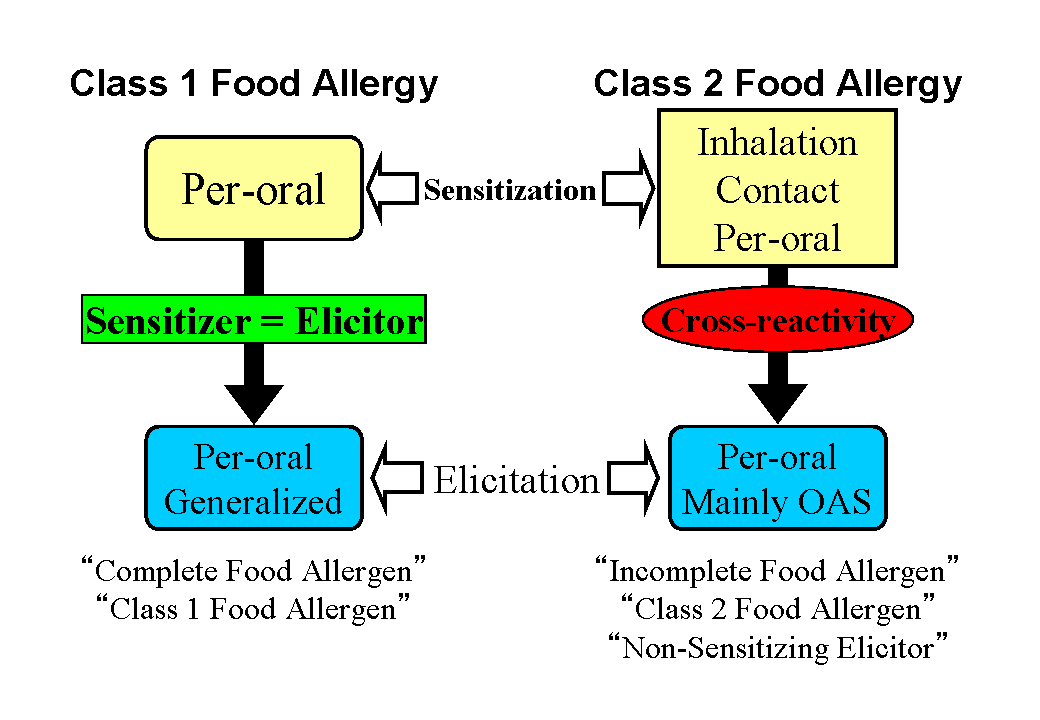 In latex-fruit syndrome and pollen-food allergy syndrome, however, distinct proteins play a vital role in the establishment of sensitization and the per-oral elicitation, respectively. A key point is the cross-reactivity of the two antigens, the sensitizer and elicitor (Fig. 3, right). Antigens in foods are only pertinent to the symptom-elicitation process and generally do not have features such as heat stability and resistance to digestive enzymes that are required to establish per-oral sensitization. It has recently been proposed that a food allergy based on the cross-reactivity between the sensitizing antigen and the symptom-eliciting antigen are called "class 2 food allergy" (or type II food allergy). In addition, food proteins responsible for such food allergies are being call "incomplete food allergens", "class 2
food allergens", or "non-sensitizing elicitors" (Fig. 3, right) [7]. An incomplete food allergen is an antigen having the capacity to elicit symptoms in already sensitized patients based on its cross-reactivity to the corresponding sensitizer. It does not have the capacity to sensitize people per-orally.
In latex-fruit syndrome and pollen-food allergy syndrome, however, distinct proteins play a vital role in the establishment of sensitization and the per-oral elicitation, respectively. A key point is the cross-reactivity of the two antigens, the sensitizer and elicitor (Fig. 3, right). Antigens in foods are only pertinent to the symptom-elicitation process and generally do not have features such as heat stability and resistance to digestive enzymes that are required to establish per-oral sensitization. It has recently been proposed that a food allergy based on the cross-reactivity between the sensitizing antigen and the symptom-eliciting antigen are called "class 2 food allergy" (or type II food allergy). In addition, food proteins responsible for such food allergies are being call "incomplete food allergens", "class 2
food allergens", or "non-sensitizing elicitors" (Fig. 3, right) [7]. An incomplete food allergen is an antigen having the capacity to elicit symptoms in already sensitized patients based on its cross-reactivity to the corresponding sensitizer. It does not have the capacity to sensitize people per-orally.
The manifestation of a class 2 food allergy is usually confined to allergic reactions around the oral cavity (namely oral allergy syndrome: OAS), likely due to the general instability of the incomplete food allergen. The empirically well-known high antigenicity of fresh fruits and vegetables compared to that of cooked or heat-treated vegetable foods can also be explained by the vulnerability of the incomplete food allergen. Although the latex-fruit syndrome is considered to be a class 2 food allergy, it must be remembered that generalized symptoms are sometimes provoked in addition to OAS. Cross-reactive food allergens relevant to this syndrome, class I chitinase, for example, are stable to some extent and can reach the intestine without complete fragmentation.
List of the Suspected Vegetables
banana, avocado, potato, tomato, kiwi, chestnut, walnut, passion fruit, pear, grapefruit, mushroom, bell pepper, mango, pineapple, celery, cantaloupe, apple, papaya, almond, buckwheat, fig, lettuce, peach, orange, peanut, strawberry, pepper, mustard, watermelon, bamboo shoot, carrot, coconut, apricot, loquat, peppermint, soybean, cherry, nectarine, .....
List of the Suspected Tree and Weed Pollen
ragweed, mugwort, timothy, Kentucky blue grass, .....
List of the Suspected Medical Plants
Condurango bark, Cannabis, .....
References
[1] Beezhold, D.H., Sussman, G.L., Liss, G.M. and Chang, N.-S.: Latex allergy can induce clinical reactions to specific foods., Clin. Exp. Allergy, 26, 416-422 (1996); Brehler, R., Theissen, U., Mohr, C. and Luger, T.: "Latex-fruit syndrome": frequency of cross-reacting IgE antibodies., Allergy, 52, 404-410 (1997).
[2] Wagner, S. and Breiteneder, H.: The latex-fruit syndrome., Biochem. Soc. Trans., 6, 935-940 (2002); Blanco, C.: Latex-fruit syndrome., Curr. Allergy Asthma Rep., 3, 47-53 (2003); Wagner, S., Radauer, C., Hafner, C., Fuchs, H., Jensen-Jarolim, E., Wuthrich, B., Scheiner, O. and Breiteneder, H.: Characterization of cross-reactive bell pepper allergens involved in the latex-fruit syndrome., Clin Exp Allergy, 34, 1739-1746 (2004).
[3] Breiteneder, H.: Plant-food and seafood allergens - an overview., Allergy, 53 (Suppl 46), 31-34 (1998); Vieths, S., Scheurer, S. and Ballmer-Weber, B.: Current understanding of cross-reactivity of food allergens and pollen., Ann. N. Y. Acad. Sci., 964, 47-68 (2002); "Plant Food Allergens", ed. by Mills, E.N.C. and Shewry, P.R., Blackwell Publishing (2003). ISBN 0-632-05982-6; Mari, A., Ballmer-Weber, B.K. and Vieths, S.: The oral
allergy syndrome: improved diagnostic and treatment methods., Curr. Opin. Allergy Clin. Immunol., 5, 267-273 (2005).
[4] Scheiner, O., Aberer, W., Ebner, C., Ferreira, F., Hoffmann-Sommergruber, K., Hsieh, L.S., Kraft, D., Sowka, S., Vanek-Krebitz, M. and Breiteneder, H.: Cross-racting allergens in tree pollen and pollen-related food allergy: implications for diagnosis of specific IgE., Int. Arch. Allergy Immunol., 113, 105-108 (1997); Salcedo, G., Diaz-Perales, A. and Sanchez-Monge, R.: The role of plant panallergens in sensitization to natural rubber latex., Curr. Opin. Allergy Clin. Immunol., 1, 177-183 (2001); van Ree R.: Clinical importance of cross-reactivity in food allergy., Curr. Opin. Allergy Clin. Immunol., 4, 235-240 (2004).
[5] Diaz-Perales, A., et al.: Class I chitinases with hevein-like domain, but not class II enzymes, are relevant chestnut and avocado allergens., J. Allergy Clin. Immunol., 102, 127-133 (1998); Chen, Z., et al.: Identification of hevein (Hev b 6.02) in Hevea latex as a major cross-reacting allergen with avocado fruit in patients with latex allergy., J. Allergy Clin. Immunol., 102, 476-481 (1998); Sowka, S., et al.: Identification and cloning of Prs a 1, a 32-kD endochitinase and major allergen of
avocado, and its expression in the yeast Pichia pastoris., J. Biol. Chem., 273, 28091-28097 (1998); Mikkola, J.H., et al.: Hevein-like protein domains as a possible cause for allergen cross-reactivity between latex and banana., J. Allergy Clin. Immunol., 102, 1005-1012 (1998); Blanco, C., et al.: Class I chitinases as potentioal panallergens involved in the latex-fruit syndrome., J. Allergy Clin. Immunol., 103, 507-513 (1999); Posch, A., et al.: Class I endochitinase containing
a hevein domain is the causative allergen in latex-associated avocado allergy., Clin. Exp. Allergy, 29, 667-672 (1999); Sanchez-Monge, R., et al.: Isolation and characterization of major banana allergens: identification as fruit class I chitinases., Clin. Exp. Allergy, 29, 673-680 (1999); Diaz-Perales, A., et al.: Cross-reactions in the latex-fruit syndrome: A relevant role of chitinases but not of complex asparagine-linked glycans. J. Allergy Clin. Immunol., 104, 681-687 (1999); Sanchez-Monge R, et al.: Class I chitinases, the panallergens responsible for the latex-fruit syndrome, are induced by ethylene treatment and inactivated by heating., J. Allergy Clin. Immunol., 106, 190-195 (2000); Diaz-Perales A, et al.:
Analysis of avocado allergen (Prs a 1) IgE-binding peptides generated by simulated gastric fluid digestion., J. Allergy Clin. Immunol., 112, 1002-1007 (2003).
[6] Seppala, U., Alenius, H., Turjanmaa, K., Reunala, T., Palosuo, T. and Kalkkinen, N.: Identification of patatin as a novel allergen for children with positive skin prick test responses to raw potato., J. Allergy Clin. Immunol., 103, 165-171 (1999); Seppala, U., Palosuo, T., Seppala, U., Kalkkinen, N., Ylitalo, L., Reunala, T., Turjanmaa, K. and Reunala T.: IgE reactivity to patatin-like latex allergen, Hev b 7, and to patatin of potato tuber, Sol t 1, in adults and children allergic to natural rubber latex., Allergy, 55, 266-273 (2000); Majamaa, H., Seppala, U., Palosuo, T., Turjanmaa, K., Kalkkinen, N. and Reunala, T.: Positive skin and oral challenge responses to potato and occurrence of immunoglobulin E antibodies to patatin (Sol t 1) in infants with atopic dermatitis. Pediatr Allergy Immunol, 12, 283-288 (2001); Schmidt, M.H., Raulf-Heimsoth, M. and Posch, A.: Evaluation of patatin as a major cross-reactive allergen in latex-induced potato allergy., Ann. Allergy Asthma Immunol., 89,613-618 (2002).
[7] Breiteneder, H. and Ebner, C.: Molecular and biochemical classification of plant-derived food allergens., J. Allergy Clin. Immunol., 106, 27-36 (2000); Aalberse, R.C.: Structural biology of allergens., J. Allergy Clin. Immunol., 106, 228-238 (2000); Bohle, B. and Vieths, S.: Improving diagnostic tests for food allergy with recombinant allergens., Methods, 32, 292-299 (2004).
Defense-Related Proteins of Higher Plants
Latex Allergens
Allergenomics (Rapid and Comprehensive Analysis of Putative Allergens)
Go to the home page of DMD
NIHS / DMD / yagami@nihs.go.jp
 In support of this assumption, the class I chitinase of avocado, chestnut, and banana, which belong to a family of pathogenesis-related proteins (PR-3), was revealed to be an important cross-reactive allergen for latex-sensitized people [5]. Class I chitinase of plants contains a hevein-like domain in common at the N-terminus (Fig. 1). Hevein (Hev b 6.02) is one of the most important latex allergens. Thus, the cross-reactivity of latex-allergic patients can be partly ascribed to the shared hevein-related structures (Fig. 1). The three-dimensional structure of hevein is shown in Fig. 2. The yellow portion indicates the linear IgE epitopes. This site closely overlaps the conserved residues indispensable to the chitin-binding
property of hevein, which is the root of its anti-fungal activity. On the other hand, patatin has been identified as a major cross-reactive allergen in potato. Patatin is a defense-related protein with lipid acyl-hydrolase or esterase activity, and functions as an important storage protein in potato tuber. Latex allergens (Hev b 7) homologous to patatin have also been found in natural rubber and have been officially registered. As in the case of cross-reactivity between class I chitinase in vegetables and hevein, the structural similarity between patatin and Hev b 7 likely accounts for part of the cross-reactivity of latex-sensitized people. [5]
In support of this assumption, the class I chitinase of avocado, chestnut, and banana, which belong to a family of pathogenesis-related proteins (PR-3), was revealed to be an important cross-reactive allergen for latex-sensitized people [5]. Class I chitinase of plants contains a hevein-like domain in common at the N-terminus (Fig. 1). Hevein (Hev b 6.02) is one of the most important latex allergens. Thus, the cross-reactivity of latex-allergic patients can be partly ascribed to the shared hevein-related structures (Fig. 1). The three-dimensional structure of hevein is shown in Fig. 2. The yellow portion indicates the linear IgE epitopes. This site closely overlaps the conserved residues indispensable to the chitin-binding
property of hevein, which is the root of its anti-fungal activity. On the other hand, patatin has been identified as a major cross-reactive allergen in potato. Patatin is a defense-related protein with lipid acyl-hydrolase or esterase activity, and functions as an important storage protein in potato tuber. Latex allergens (Hev b 7) homologous to patatin have also been found in natural rubber and have been officially registered. As in the case of cross-reactivity between class I chitinase in vegetables and hevein, the structural similarity between patatin and Hev b 7 likely accounts for part of the cross-reactivity of latex-sensitized people. [5] It is estimated that 50-70 % of latex-allergic people have IgE antibodies cross-reactive to the antigens coming from some vegetable foods [1]. In addition, increasing numbers of plant-derived foods are suspected to cross-react with latex antigens. Fruits are especially notorious for their frequent cross-reactivity, which is referred to as "latex-fruit syndrome"[2,3]. Besides various plant-derived foods, latex-allergic people are occasionally affected by some kinds of pollen and medical plants. This extensive cross-reactivity of latex-allergic patients is explained mostly by the major latex allergens are being pathogenesis-, or rather, defense-related proteins of a rubber tree. Higher plants universally
induce such protective proteins under certain conditions, and their structures are relatively conserved during the course of evolution. These features of defense-related proteins provide common epitopes for IgE antibodies [4]. In other words, it is considered that the defense-related proteins of the rubber tree and of cross-reactive plants act as pan-allergens responsible for the extensive cross-reactivity of latex-sensitized people.
It is estimated that 50-70 % of latex-allergic people have IgE antibodies cross-reactive to the antigens coming from some vegetable foods [1]. In addition, increasing numbers of plant-derived foods are suspected to cross-react with latex antigens. Fruits are especially notorious for their frequent cross-reactivity, which is referred to as "latex-fruit syndrome"[2,3]. Besides various plant-derived foods, latex-allergic people are occasionally affected by some kinds of pollen and medical plants. This extensive cross-reactivity of latex-allergic patients is explained mostly by the major latex allergens are being pathogenesis-, or rather, defense-related proteins of a rubber tree. Higher plants universally
induce such protective proteins under certain conditions, and their structures are relatively conserved during the course of evolution. These features of defense-related proteins provide common epitopes for IgE antibodies [4]. In other words, it is considered that the defense-related proteins of the rubber tree and of cross-reactive plants act as pan-allergens responsible for the extensive cross-reactivity of latex-sensitized people.