Defense-Related Proteins of Higher Plants
[ English | Japanese ]
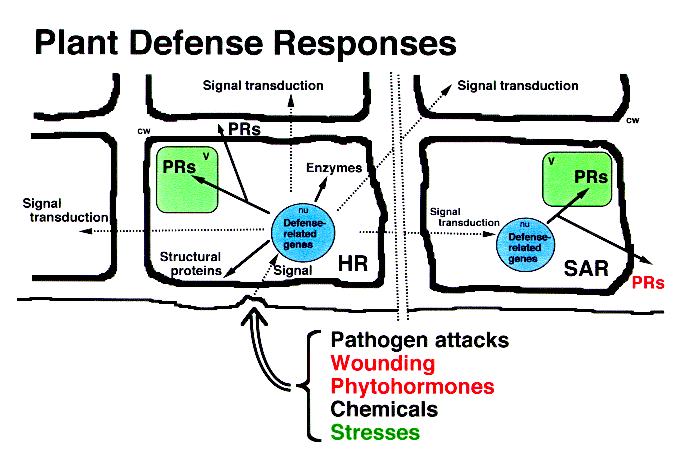 By changing their physiological conditions, higher plants protect themselves from various stresses such as pathogen attacks, wounding, application of chemicals including phytohormone and heavy metals, air pollutants like ozone, ultraviolet rays, and harsh growing conditions. These protective reactions are known as "defense responses" of higher plants (Fig. 1), and the proteins actively synthesized in accordance with this reaction are called "defense-related proteins" [1]. In particular, protective plant proteins specifically induced in pathological or related situations have been intensively studied from an agricultural perspective and are called "pathogenesis-related proteins" (PR proteins). Enzymes indispensable for biosynthesizing low-molecular-weight antibiotics (phytoalexin), isoflavone reductase, for example, can also be considered to be defense-related proteins. On the other hand, many of the reserve proteins accumulated in seeds and fruits are considered to have a constitutive defense function against microbial pathogens and invertebrate pests in addition to their storage function. These inducible or constitutive defense mechanisms of higher plants are relatively conserved during the course of evolution. Accordingly, most plants produce or accumulate structurally and functionally similar protective proteins under certain
situations, irrespective of their morphological differences. For instance, PR proteins, which have been found in many plant species to date, are classified into seventeen families (Table), regardless of the original plant species. The sequence similarities, serologic or immunologic relationships, and enzymatic properties are the basis of this classification [2].
By changing their physiological conditions, higher plants protect themselves from various stresses such as pathogen attacks, wounding, application of chemicals including phytohormone and heavy metals, air pollutants like ozone, ultraviolet rays, and harsh growing conditions. These protective reactions are known as "defense responses" of higher plants (Fig. 1), and the proteins actively synthesized in accordance with this reaction are called "defense-related proteins" [1]. In particular, protective plant proteins specifically induced in pathological or related situations have been intensively studied from an agricultural perspective and are called "pathogenesis-related proteins" (PR proteins). Enzymes indispensable for biosynthesizing low-molecular-weight antibiotics (phytoalexin), isoflavone reductase, for example, can also be considered to be defense-related proteins. On the other hand, many of the reserve proteins accumulated in seeds and fruits are considered to have a constitutive defense function against microbial pathogens and invertebrate pests in addition to their storage function. These inducible or constitutive defense mechanisms of higher plants are relatively conserved during the course of evolution. Accordingly, most plants produce or accumulate structurally and functionally similar protective proteins under certain
situations, irrespective of their morphological differences. For instance, PR proteins, which have been found in many plant species to date, are classified into seventeen families (Table), regardless of the original plant species. The sequence similarities, serologic or immunologic relationships, and enzymatic properties are the basis of this classification [2].
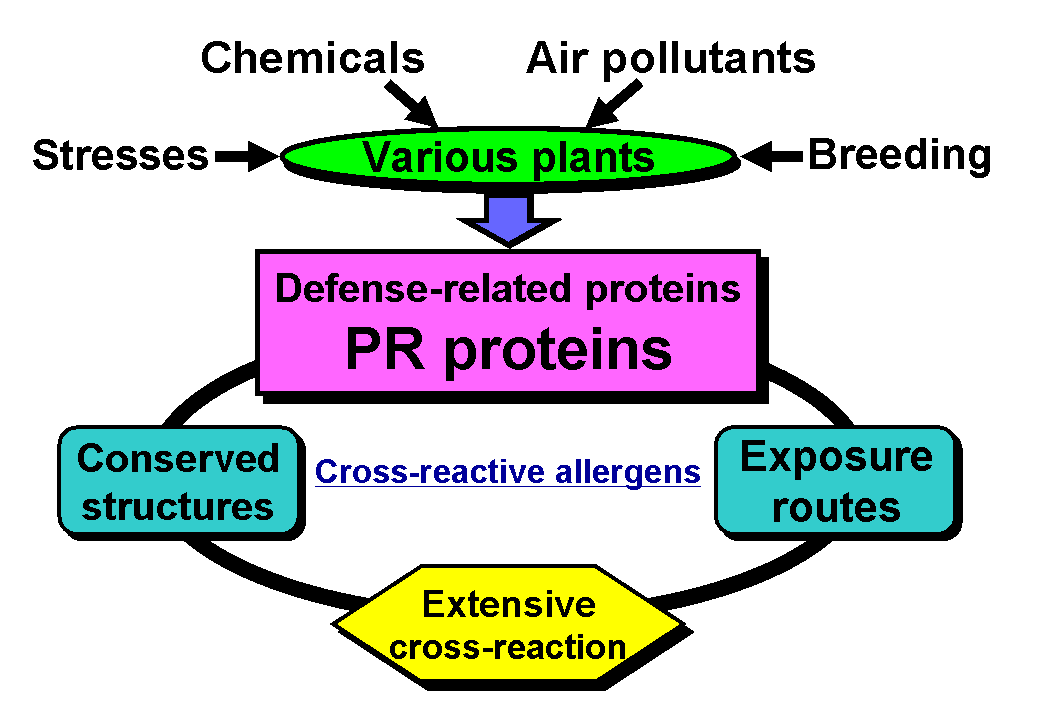 Recently, protective proteins of higher plants have drawn much attention from plant breeders [3]. Because defense-related proteins usually provide a plant with resistance to stresses, varieties that are apt to intensively induce such proteins can be agriculturally valuable. Less toxic substances that cause crops to express defensive proteins are also being investigated as a new type of agrochemical. Moreover, some defense-related proteins will be constantly expressed in genetically modified plants [4-7]. However, several of these proteins have been proven to be latex allergens and cross-reactive allergens in fruits, vegetables, and pollen [8-10]. As such, it is probable that the crop content of allergenic proteins responsible for "oral allergy syndrome" (OAS) or similar syndromes has increased in response to traditional plant-breeding or the creation of stress-resistant varieties through the use of gene recombination techniques (Fig. 2). When a genetically modified new crop is brought to market as a food, its safety, including its possible allergenicity, must be strictly examined and reviewed in advance [11].
Recently, protective proteins of higher plants have drawn much attention from plant breeders [3]. Because defense-related proteins usually provide a plant with resistance to stresses, varieties that are apt to intensively induce such proteins can be agriculturally valuable. Less toxic substances that cause crops to express defensive proteins are also being investigated as a new type of agrochemical. Moreover, some defense-related proteins will be constantly expressed in genetically modified plants [4-7]. However, several of these proteins have been proven to be latex allergens and cross-reactive allergens in fruits, vegetables, and pollen [8-10]. As such, it is probable that the crop content of allergenic proteins responsible for "oral allergy syndrome" (OAS) or similar syndromes has increased in response to traditional plant-breeding or the creation of stress-resistant varieties through the use of gene recombination techniques (Fig. 2). When a genetically modified new crop is brought to market as a food, its safety, including its possible allergenicity, must be strictly examined and reviewed in advance [11].
Table. Recommended Classification of Pathogenesis-Related Proteins (PRs)
| Family |
Type member |
Properties |
| PR-1 |
tobacco PR-1a |
antifungal?, 14-17kD |
| PR-2 |
tobacco PR-2 |
class I, II, and III endo-beta-1,3-glucanases, 25-35kD |
| PR-3 |
tobacco P, Q |
class I, II, IV, V, VI, and VII endochitinases, about 30kD |
| PR-4 |
tobacco R |
antifungal, win-like proteins, endochitinase activity,
similar to prohevein C-terminal domain, 13-19kD |
| PR-5 |
tobacco S |
antifungal, thaumatin-like proteins,
osmotins, zeamatins, permeatins,
similar to alpha-amylase/trypsin inhibitors |
| PR-6 |
tomato inhibitor I |
protease inhibitors, 6-13kD |
| PR-7 |
tomato P69 |
endoproteases |
| PR-8 |
cucumber chitinase |
class III chitinases, chitinase/lysozyme |
| PR-9 |
lignin-forming peroxidase |
peroxidases, peroxidase-like proteins |
| PR-10 |
parsley PR-1 |
ribonucleases, Bet v 1-related proteins |
| PR-11 |
tobacco class V chitinase |
endochitinase activity |
| PR-12 |
radish Ps-AFP3 |
plant defensins |
| PR-13 |
Arabidopsis THI2.1 |
thionins |
| PR-14 |
barley LTP4 |
nonspecific lipid transfer proteins (ns-LTPs) |
| PR-15 |
barley OxOa (germin) |
oxalate oxidase |
| PR-16 |
barley OxOLP |
oxalate-oxidase-like proteins |
| PR-17 |
tobacco PRp27 |
unknown |
Other plant proteins with defensive activities against pests and pathogens
2S storage albumins, patatin, ribosome-inactivating proteins (RIPs), polygalacturonase inhibitor proteins (PGIPs), nonenzymatic chitin-binding proteins (hevein, lectins, AMPs, AFPs, etc.), cystatins, prolamin
storage proteins, vicilin (7S) storage proteins, .....
References
[1] Bowles, D.J.: Defense-related proteins in higher plants., Annu. Rev. Biochem., 59, 873-907 (1990).
[2] Van Loon, L.C., Pierpoint, W.S., Boller, Th. and Conejero, V.: Recommendations for naming plant pathogenesis-related proteins., Plant Mol. Biol. Report.,12, 245-264 (1994); Van Loon, L.C. and Van Strien, E.A.: The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins., Physiol. Mol. Plant Pathol., 55, 85-97 (1999).
[3] Fritig, B., Heitz, T. and Legrand, M.: Antimicrobial proteins in induced plant defense., Curr. Opin. Immunol., 10, 16-22 (1998).
[4] Lee, H.-I. and Raikhel, N.V.: Prohevein is poorly processed but shows enhanced resistance to a chitin-binding fungus in transgenic tomato plants., Braz. J. Med. Biol. Res., 28, 743-750 (1995); Kanrara, S., Venkateswaria, J.C., Kirtib, P.B. and Chopra, V.L.: Transgenic expression of hevein, the rubber tree lectin, in Indian mustard confers protection against Alternaria brassicae., Plant Sci., 162, 441-448 (2002).
[5] Shah, D.M.: Genetic engineering for fungal and bacterial diseases., Curr. Opin. Biotechnol., 8, 208-214 (1997).
[6] Shewry, P.R. and Lucas J.A.: Plant proteins that confer resistance to pests and pathogens., Adv. Bot. Res., 26, 135-192 (1997).
[7] Datta, S.K. and Muthukrishnan, S., eds., Pathogenesis-related proteins in plants, CRC press, Washington, D.C. (1999). ISBN 0-8493-0697-3
[8] Breiteneder, H.: Plant-food and seafood allergens - an overview., Allergy, 53 (Suppl 46), 31-34 (1998); Vieths, S., Scheurer, S. and Ballmer-Weber, B.: Current understanding of cross-reactivity of food allergens and pollen., Ann. N. Y. Acad. Sci., 964, 47-68 (2002).
[9] Hanninen, A.R., et al.: Increased allergen production in turnip (Brassica rapa) by treatments activating defense mechanisms., J. Allergy Clin. Immunol., 104, 194-201 (1999); Hanninen, A.R., et al.:Prohevein-like defense protein of tobacco is a cross-reactive allergen for latex-allergic patients., J. Allergy Clin. Immunol., 106, 778-779 (2000); Armentia, A., et al.: Is Lolium pollen from an urban environment more allergenic than rural pollen?, Allergol. Immunopathol.
(Madr.), 30, 218-224 (2002); Armentia, A., et al.: Enhancement of tomato allergenicity after treatment with plant hormones., Allergol. Immunopathol. (Madr.), 31, 44-46 (2003); Krebitz, et al.: Plant-based heterologous expression of Mal d 2, a thaumatin-like protein and allergen of apple (Malus domestica), and its characterization as an antifungal protein., J. Mol. Biol., 329, 721-730 (2003); Cortegano, I., et al.: Cloning and expression of a major allergen from Cupressus arizonica
pollen, Cup a 3, a PR-5 protein expressed under polluted environment., Allergy, 59, 485-490 (2004); Chehregani, A., et al.: Increasing allergy potency of Zinnia pollen grains in polluted areas., Ecotoxicol. Environ. Saf., 58, 267-272 (2004); Tashpulatov, A.S., et al.: A model system to study the environment-dependent expression of the Bet v 1a gene encoding the major birch pollen allergen., Int. Arch. Allergy Immunol., 134, 1-9 (2004).
[10] Salcedo, G., Diaz-Perales, A. and Sanchez-Monge, R.: Fruit allergy: plant defence proteins as novel potential panallergens., Clin. Exp. Allergy., 29, 1158-1160 (1999); Breiteneder, H. and Ebner, C.: Molecular and biochemical classification of plant-derived food allergens., J. Allergy Clin. Immunol., 106, 27-36 (2000); Hoffmann-Sommergruber, K.: Plant allergens and pathogenesis-related proteins., Int. Arch. Allergy Immunol., 122, 155-166 (2000); Ebner, C., Hoffmann-Sommergruber, K. and Breiteneder,
H.: Plant food allergens homologous to pathogenesis-related proteins., Allergy, 56(Suppl.67), 43-44 (2001); Midoro-Horiuti, T., Brooks, E.G. and Goldblum, R.M.: Pathogenesis-related proteins of plants as allergens., Ann Allergy Asthma Immunol, 87, 261-271 (2001); Breiteneder, H. and Ebner, C.: Atopic allergens of plant foods., Curr. Opin. Allergy Clin. Immunol., 1, 261-267 (2001); Hoffmann-Sommergruber, K.: Pathogenesis-related (PR)-proteins identified as allergens., Biochem. Soc. Trans., 6, 930-935 (2002).
[11] Taylor, S.L.: Protein allergenicity assessment of foods produced through agricultural biotechnology., Annu. Rev. Pharmacol. Toxicol., 42, 99-112 (2002); BSACI working party: Lack, G., et al.: Report on the potential allergenicity of genetically modified organisms and their products., Clin. Exp. Allergy, 32, 1131-1143 (2002); Fu, T.J., Abbott, U.R. and Hatzos, C.: Digestibility of food allergens and nonallergenic proteins in simulated gastric fluid and simulated intestinal fluid - A comparative study.,
J. Agric. Food Chem., 50, 7154-7160 (2002); Brusic, V. and Petrovsky, N.: Bioinformatics for characterisation of allergens, allergenicity and allergic crossreactivity., Trends Immunol., 24, 225-228 (2003); Fiers, M. W.E.J., et al.,: Allermatch, a webtool for the prediction of potential allergenicity according to current FAO/WHO Codex alimentarius guidelines., BMC Bioinformatics, 5(1), 133 (2004); Jenkins, J.A., et al.; Structural relatedness of plant food allergens with specific reference to
cross-reactive allergens: an in silico analysis., J. Allergy Clin. Immunol., 115163-170 (2005).
Latex-Fruit Syndrome and Class 2 Food Allergy
Latex Allergens
Allergenomics (Rapid and Comprehensive Analysis of Putative Allergens)
Go to the home page of DMD
NIHS / DMD / yagami@nihs.go.jp
 Recently, protective proteins of higher plants have drawn much attention from plant breeders [3]. Because defense-related proteins usually provide a plant with resistance to stresses, varieties that are apt to intensively induce such proteins can be agriculturally valuable. Less toxic substances that cause crops to express defensive proteins are also being investigated as a new type of agrochemical. Moreover, some defense-related proteins will be constantly expressed in genetically modified plants [4-7]. However, several of these proteins have been proven to be latex allergens and cross-reactive allergens in fruits, vegetables, and pollen [8-10]. As such, it is probable that the crop content of allergenic proteins responsible for "oral allergy syndrome" (OAS) or similar syndromes has increased in response to traditional plant-breeding or the creation of stress-resistant varieties through the use of gene recombination techniques (Fig. 2). When a genetically modified new crop is brought to market as a food, its safety, including its possible allergenicity, must be strictly examined and reviewed in advance [11].
Recently, protective proteins of higher plants have drawn much attention from plant breeders [3]. Because defense-related proteins usually provide a plant with resistance to stresses, varieties that are apt to intensively induce such proteins can be agriculturally valuable. Less toxic substances that cause crops to express defensive proteins are also being investigated as a new type of agrochemical. Moreover, some defense-related proteins will be constantly expressed in genetically modified plants [4-7]. However, several of these proteins have been proven to be latex allergens and cross-reactive allergens in fruits, vegetables, and pollen [8-10]. As such, it is probable that the crop content of allergenic proteins responsible for "oral allergy syndrome" (OAS) or similar syndromes has increased in response to traditional plant-breeding or the creation of stress-resistant varieties through the use of gene recombination techniques (Fig. 2). When a genetically modified new crop is brought to market as a food, its safety, including its possible allergenicity, must be strictly examined and reviewed in advance [11].
 By changing their physiological conditions, higher plants protect themselves from various stresses such as pathogen attacks, wounding, application of chemicals including phytohormone and heavy metals, air pollutants like ozone, ultraviolet rays, and harsh growing conditions. These protective reactions are known as "defense responses" of higher plants (Fig. 1), and the proteins actively synthesized in accordance with this reaction are called "defense-related proteins" [1]. In particular, protective plant proteins specifically induced in pathological or related situations have been intensively studied from an agricultural perspective and are called "pathogenesis-related proteins" (PR proteins). Enzymes indispensable for biosynthesizing low-molecular-weight antibiotics (phytoalexin), isoflavone reductase, for example, can also be considered to be defense-related proteins. On the other hand, many of the reserve proteins accumulated in seeds and fruits are considered to have a constitutive defense function against microbial pathogens and invertebrate pests in addition to their storage function. These inducible or constitutive defense mechanisms of higher plants are relatively conserved during the course of evolution. Accordingly, most plants produce or accumulate structurally and functionally similar protective proteins under certain
situations, irrespective of their morphological differences. For instance, PR proteins, which have been found in many plant species to date, are classified into seventeen families (Table), regardless of the original plant species. The sequence similarities, serologic or immunologic relationships, and enzymatic properties are the basis of this classification [2].
By changing their physiological conditions, higher plants protect themselves from various stresses such as pathogen attacks, wounding, application of chemicals including phytohormone and heavy metals, air pollutants like ozone, ultraviolet rays, and harsh growing conditions. These protective reactions are known as "defense responses" of higher plants (Fig. 1), and the proteins actively synthesized in accordance with this reaction are called "defense-related proteins" [1]. In particular, protective plant proteins specifically induced in pathological or related situations have been intensively studied from an agricultural perspective and are called "pathogenesis-related proteins" (PR proteins). Enzymes indispensable for biosynthesizing low-molecular-weight antibiotics (phytoalexin), isoflavone reductase, for example, can also be considered to be defense-related proteins. On the other hand, many of the reserve proteins accumulated in seeds and fruits are considered to have a constitutive defense function against microbial pathogens and invertebrate pests in addition to their storage function. These inducible or constitutive defense mechanisms of higher plants are relatively conserved during the course of evolution. Accordingly, most plants produce or accumulate structurally and functionally similar protective proteins under certain
situations, irrespective of their morphological differences. For instance, PR proteins, which have been found in many plant species to date, are classified into seventeen families (Table), regardless of the original plant species. The sequence similarities, serologic or immunologic relationships, and enzymatic properties are the basis of this classification [2].